
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The nucleus is composed of protons and neutrons. ISBN 978-0-8493-0485-9.The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. CRC Handbook of Chemistry and Physics (85th ed.). Half-life, spin, and isomer data selected from the following sources.International Union of Pure and Applied Chemistry. "News & Notices: Standard Atomic Weights Revised"."Atomic weights of the elements 2005 (IUPAC Technical Report)". de Laeter, John Robert Böhlke, John Karl De Bièvre, Paul Hidaka, Hiroshi Peiser, H.Isotopic compositions and standard atomic masses from:.Audi, Georges Bersillon, Olivier Blachot, Jean Wapstra, Aaldert Hendrik (2003), "The N UBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode: 2003NuPhA.729.3A, doi: 10.1016/j.nuclphysa.2003.11.001."Production cross sections from 82 Se fragmentation as indications of shell effects in neutron-rich isotopes close to the drip-line". "Evidence for a Change in the Nuclear Mass Surface with the Discovery of the Most Neutron-Rich Nuclei with 17 ≤ Z ≤ 25". "Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes".

"Standard atomic weights of the elements 2021 (IUPAC Technical Report)". ^ Prohaska, Thomas Irrgeher, Johanna Benefield, Jacqueline et al.^ "Standard Atomic Weights: Chromium"."The NUBASE2020 evaluation of nuclear properties" (PDF). It is used as a diagnostic radiopharmaceutical agent in nephrology to determine glomerular filtration rate, and in hematology to determine red blood cell volume or mass, study the red blood cell survival time and evaluate blood loss. Chromium Cr-51 has been used as a radioactive label for decades. ^ a b c d e f g h i Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide.Ĭhromium-51 is a synthetic radioactive isotope of chromium having a half-life of 27.7 days and decaying by electron capture with emission of gamma rays (0.32 MeV) it is used to label red blood cells for measurement of mass or volume, survival time, and sequestration studies, for the diagnosis of gastrointestinal bleeding, and to label platelets to study their survival.^ Suspected of decaying by double electron capture to 50Ti with a half-life of no less than 1.3 ×10 18 a.^ ( ) spin value – Indicates spin with weak assignment arguments.^ Bold symbol as daughter – Daughter product is stable.^ a b c # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).^ # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).^ ( ) – Uncertainty (1 σ) is given in concise form in parentheses after the corresponding last digits.The primary decay mode before the most abundant stable isotope, 52Cr, is electron capture and the primary mode after is beta decay. The isotopes of chromium range from 42Cr to 70Cr. The same isotope is preferentially involved in certain leaching reactions, thereby allowing its abundance in seawater sediments to be used as a proxy for atmospheric oxygen concentrations. Hence 53Cr provides additional evidence for nucleosynthetic processes immediately before coalescence of the Solar System. Variations in 53Cr/ 52Cr and Mn/Cr ratios from several meteorites indicate an initial 53Mn/ 55Mn ratio that suggests Mn-Cr isotope systematics must result from in-situ decay of 53Mn in differentiated planetary bodies. Mn-Cr isotope ratios reinforce the evidence from 26Al and 107 Pd for the early history of the Solar System.
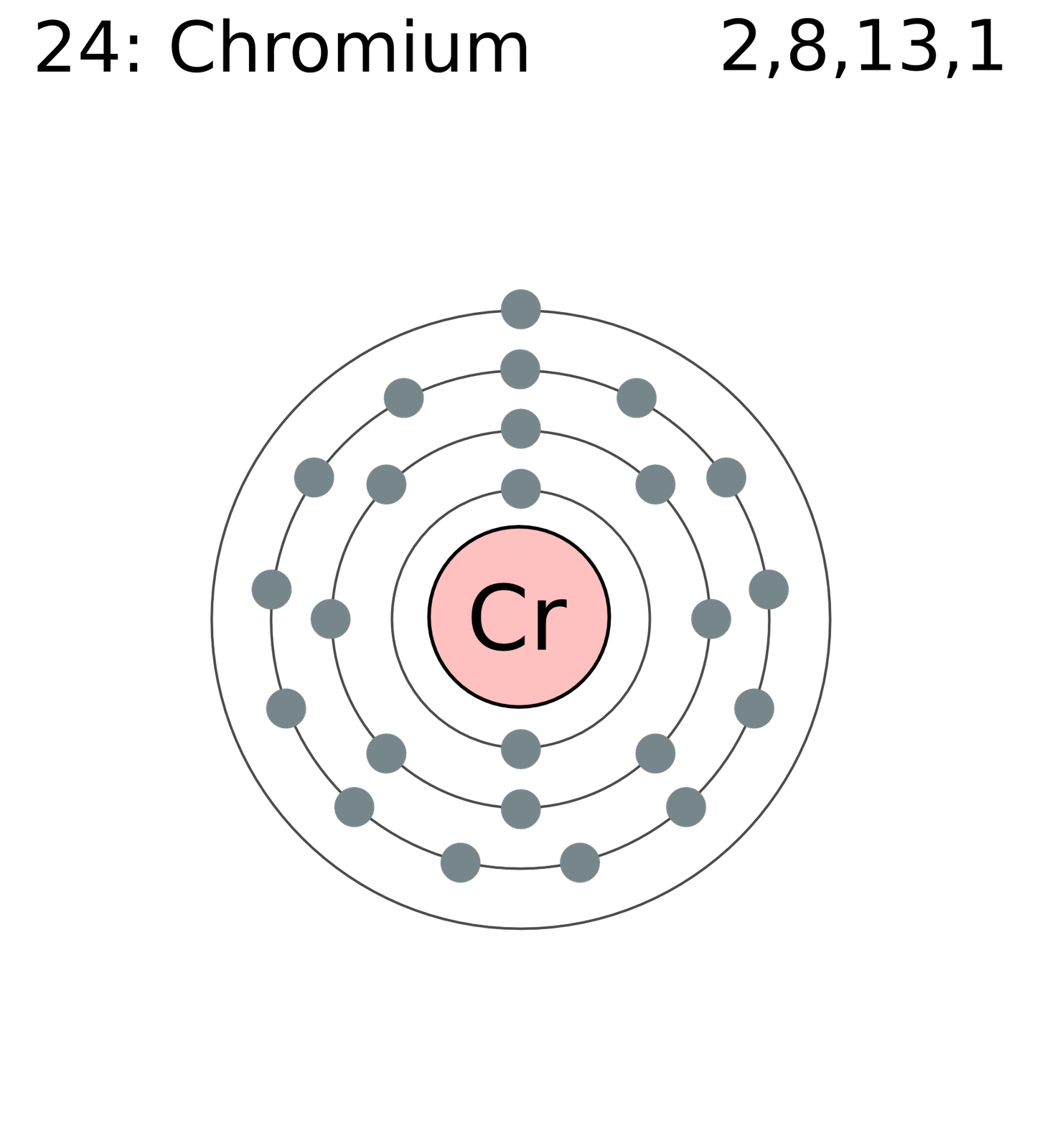
Chromium isotopic contents are typically combined with manganese isotopic contents and have found application in isotope geology. This element also has two meta states, 45mCr, the more stable one, and 59mCr, the least stable isotope or isomer.ĥ3Cr is the radiogenic decay product of 53 Mn. All of the remaining radioactive isotopes have half-lives that are less than 24 hours and the majority of these have half-lives that are less than 1 minute. Twenty-two radioisotopes, all of which are entirely synthetic, have been characterized, the most stable being 51Cr with a half-life of 27.7 days. 50Cr is suspected of decaying by β +β + to 50Ti with a half-life of (more than) 1.8×10 17 years. Naturally occurring chromium ( 24Cr) is composed of four stable isotopes 50Cr, 52Cr, 53Cr, and 54Cr with 52Cr being the most abundant (83.789% natural abundance).


 0 kommentar(er)
0 kommentar(er)
